
Indication
LEQEMBI is approved for the treatment of early onset Alzheimer’s diseases, mild cognitive impairment or mild dementia. The FDA gave LEQEMBI approval under the accelerated approval. This was based on the reduction of the amyloid beta plaques observed in patients
Mechanism of action
LEQEMBI binds selectively to soluble Aβ protofibrils, rather than monomers or mature plaques.
- Protofibrils are intermediate aggregates of Aβ that are particularly neurotoxic and contribute to synaptic dysfunction and neuronal death.
Immune-mediated Clearance: Once bound, lecanemab flags the protofibrils for clearance by microglia, the brain’s immune cells.
- This immune response helps reduce amyloid burden in the brain over time.
DRUG DELIVERY/Dosage Regiment
Amount: 10 mg/kg administered intravenously every two weeks
Route: Intravenous infusion.
Infusion time: Administered over ~1 hour.
No titration: Unlike some drugs, LEQEMBI is started at the full dose from the beginning—there is no dose escalation phase.
Monitoring: Brain MRI scans are typically conducted before starting treatment and periodically during therapy to monitor for ARIA (Amyloid-Related Imaging Abnormalities).
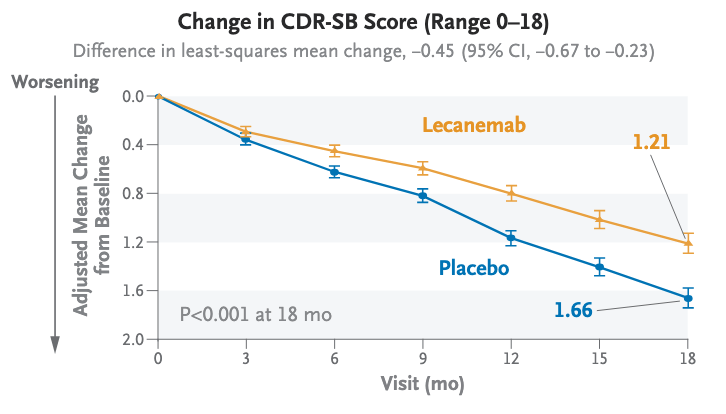
Source – van Dyck, C. H., et al. (2023). Lecanemab in early Alzheimer’s disease. New England Journal of Medicine, 388(1), 9–21. https://doi.org/10.1056/NEJMoa2212948
Example of clinical evidence for LEQEMBI in Phase 3. The above data is from a Phase 3 study, multicenter, double-blind, randomized, placebo-controlled trial assessed the efficacy and
safety of lecanemab in patients 50 to 90 years of age with early Alzheimer’s disease.
At 18 months Participants receiving lecanemab had a 27% slower decline in cognitive scores for linical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months compared to placebo. Amyloid PET imaging showed significant reduction in brain amyloid.
COST
Estimated around $27,000 per year at full maintenance of the dosing regiment in a year as of 2024
Covered by Medicare in the United States but patients are responsible for co-pay.
Availability
Countries LEQEMBI is approved and available as of May 2025
- United States
- Japan
- China
- South Korea
- Hong Kong
- Israel
- United Arab Emirates
- Great Britain
- Mexico
- Macau
Leave a comment