Overview
In relatively recent years, it has come to the attention of many scientists and companies alike, that microglia hold the secrets to further understanding neurodegenerative disease progression. In this post, I will cover the main findings, the cells that are at play, some of the relevant genes at play and future outlook for research in this area.
Diving In
The immune system has been connected with being the main driver of inflammatory conditions and autoimmune diseases. There is strong research showing that the immune system plays a significant role in neurodegenerative diseases. The central player here is the microglia.
Microglia are the primary innate immune cells of the central nervous system and play a critical role in the pathogenesis of Alzheimer’s disease (AD). Their functions in AD are multifaceted, encompassing protective and detrimental effects, which vary across disease progression.
Amyloid-β (Aβ) Clearance and Phagocytosis
Microglia recognize and phagocytose Aβ peptides via surface receptors such as TLRs, CD36, and TREM2. In early AD, this contributes to plaque clearance and neuroprotection. However, chronic exposure to Aβ leads to impaired phagocytic efficiency and promotes a pro-inflammatory state. By recognizing and engulfing Aβ plaques, a hallmark of AD – early in the disease, this clearance provides protection.
Neuroinflammation and Cytokine Release
Sustained microglial activation releases pro-inflammatory cytokines (e.g., IL-1β, TNF-α, IL-6) and reactive oxygen species (ROS). This neuroinflammatory environment contributes to neuronal dysfunction, tau hyperphosphorylation, and synaptic loss, exacerbating disease pathology. Chronic activation of microglia leads to sustained neuroinflammation. They release cytokines and reactive oxygen species that can damage neurons and exacerbate disease progression
Synaptic Pruning and Dysfunction
Microglia are involved in activity-dependent synaptic pruning through complement signaling pathways (e.g., C1q, C3). In AD, aberrant activation of these pathways leads to excessive synaptic elimination, correlating with cognitive decline.
Modulation of Tau Pathology
Microglial inflammation promotes tau pathology through indirect mechanisms. Pro-inflammatory cytokines can enhance tau phosphorylation via kinases such as GSK3β and CDK5, and activated microglia may facilitate the spread of tau aggregates between neurons.
Genetic Risk and TREM2 Signaling
Variants in microglial genes, notably TREM2, significantly impact AD risk. TREM2 modulates microglial metabolism, survival, and response to Aβ. Loss-of-function mutations (e.g., R47H) impair microglial ability to cluster around plaques and contain pathology.
Phenotypic Heterogeneity
Microglia exhibit a spectrum of activation states in AD. Recent single-cell transcriptomic studies have identified disease-associated microglia (DAM) subpopulations characterized by upregulation of genes such as Apoe, Lpl, and Trem2, suggesting a transition toward a neurodegeneration-associated phenotype.
In conclusion, microglia orchestrate protective and deleterious responses in AD through a network of phagocytic, inflammatory, and regulatory pathways. Therapeutic strategies targeting microglial function—such as TREM2 agonists or inflammatory signaling modulators—are promising in modifying disease progression.
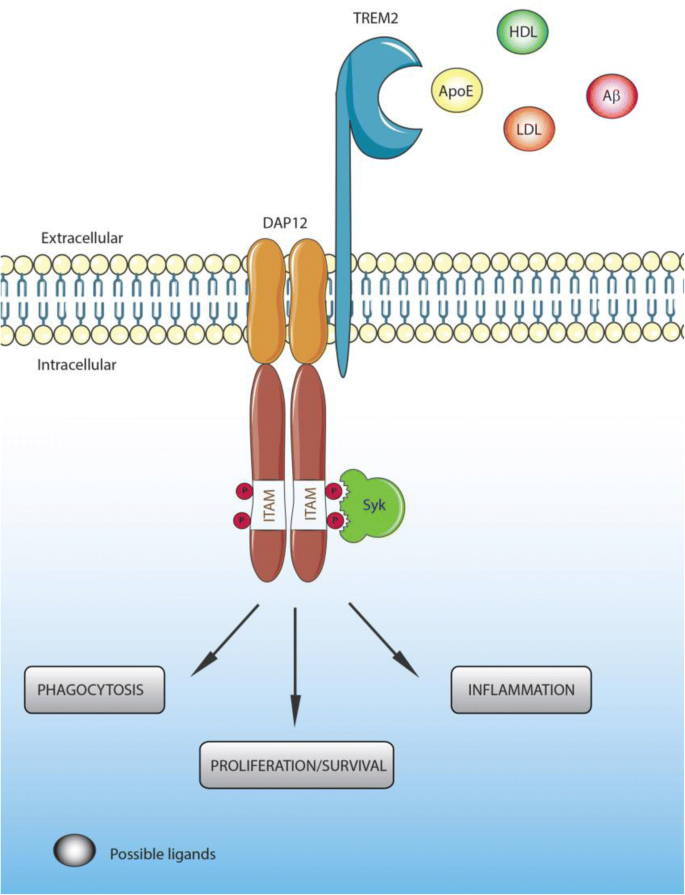
The microglial receptor TREM2 is critical in modulating microglial response to AD pathology. Variants in TREM2 are associated with increased AD risk, highlighting its regulatory role.
Leave a comment