
Baby KJ has already become a celebrity and will go down in history books, and he is way too young to know any of it. In February of 2025, he received first-in-class therapy for treating a rare genetic disorder that has been successfully treated using a customized CRISPR gene-editing therapy developed by a team at the Children’s Hospital of Philadelphia (CHOP) and Penn Medicine. The infant, KJ, was born with severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, a rare metabolic condition. This condition prevented him from breaking down proteins in his food. For most patients with this disease, their only hope is to get a liver transplant. For KJ, at such a young age, an organ transplant is very risky. After spending his first several months in the hospital on a highly restricted diet, KJ received his first dose of the personalized therapy in February at around six to seven months old. The treatment was safely administered, and he is now healthy, growing well, and thriving.

This customized therapy was developed with a vast team that was comprised of physician-scientists at Children’s Hospital of Philadelphia (CHOP), a research team at Innovative Genomics Institute (IGI) at the University of California, Berkeley, scientists at the Jackson Laboratory, and industry partners who are Aldevron, Integrated DNA Technologies (IDT) and Acuitas Therapeutics. The multi-institutional team worked together to design, test, manufacture, and navigate regulatory review with the U.S. Food and Drug Administration (FDA), ensuring the therapy could be delivered as swiftly as possible. The shocker is that this all came together in just six months. Regarding how long drug discovery takes- bench-level insight, R&D, animal testing, filing for IND with the FDA, and clinical testing – six months is a minuscule amount of time. Though the pieces came together swiftly, this is not to say the individual partners on this team weren’t involved with CRISPR therapies or had scientific knowledge of the urea cycle disorder.
Since its founding in 2015, the Innovative Genomics Institute (IGI) has made developing CRISPR-based treatments for rare diseases a core part of its mission. Over the past two years, the IGI-Danaher Beacon for CRISPR Cures project has been creating a “cookbook” for designing and producing on-demand CRISPR therapies. In parallel, IGI labs have been working on platform-based CRISPR treatment strategies and developing safety assays for gene-editing therapies—efforts supported by the NIH’s Somatic Cell Genome Editing program. This NIH initiative also facilitated a connection between IGI researchers and the Children’s Hospital of Philadelphia (CHOP) team and the University of Pennsylvania, including study lead Dr. Rebecca Ahrens-Nicklas. In early August 2024, Drs. Kiran Musunuru and Ahrens-Nicklas shared with IGI’s Fyodor Urnov the clinical story of a CHOP patient suffering from a urea cycle disorder caused by a known genetic mutation but lacking a cure. This story inspired Urnov and the Beacon for the CRISPR Cures project.
Another reason why KJ could be treated so quickly was that his case was a good place to try this new therapy. This was because the team could enroll KJ into IGI quickly for genome sequencing analysis, and his mutation was a single base, one-letter change in his genetic code. Which also made designing the gene editing techniques much easier. Additionally, the researchers needed to target and edit cells in the liver, where the defective protein is produced. The liver is one of the most accessible organs for in vivo gene-editing therapies using lipid nanoparticles.
Future outlook for crispr therapies
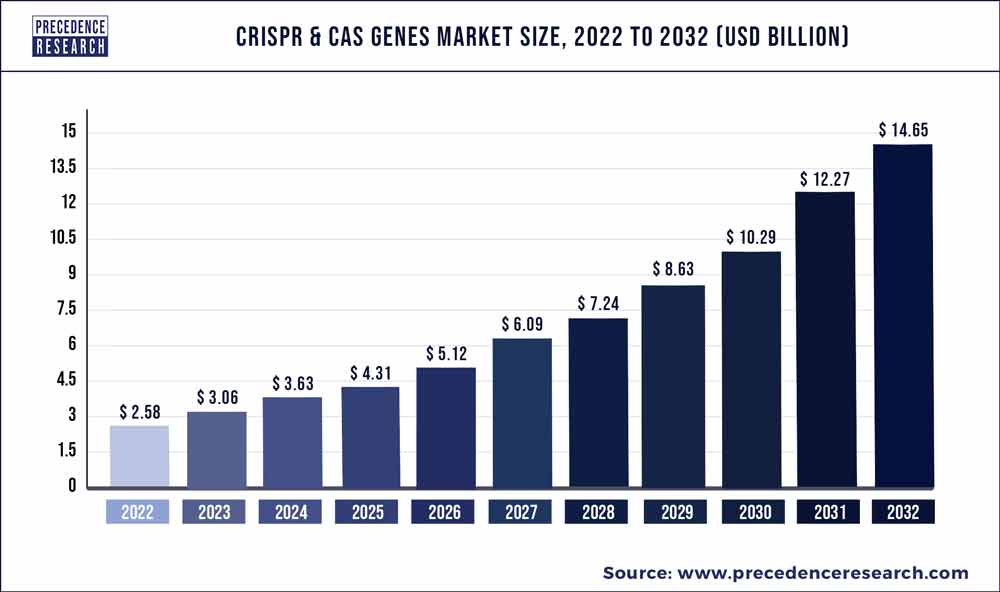
According to BioSpace, the global CRISPR & Cas genes market size was valued at $2.58 billion in 2022 and is projected to reach at least $14.65 billion by 2032 with an estimated CAGR of 19%.
The use of CRISPR in drug development and therapeutic applications has been limited by its potential to cause off-target effects—unintended genetic changes that pose a significant challenge for human gene editing. These rare but critical errors can compromise safety and effectiveness in clinical settings. However, the recent discovery of anti-CRISPR proteins offers a promising strategy to mitigate these risks, improving the accuracy and safety of CRISPR for both research and therapeutic use.
North America’s strong position in the global economy has made it a leader in CRISPR adoption. Federal initiatives in the U.S. and Canada actively support research in agricultural biotechnology and the commercialization of CRISPR-edited crops. A notable boost for CRISPR’s agricultural use came when the USDA opted not to regulate the first CRISPR-edited soybean products. Additionally, substantial investments from pharmaceutical and seed companies—through acquisitions, partnerships, and collaborations—are accelerating growth in both the pharmaceutical and agricultural sectors, driving further demand for CRISPR technology.
Favorable regulatory policies have also increased the prevalence of clinical trials and CRISPR drug developments in North America. In 2023, the U.S. FDA approved Casgevy and Lyfgenia, marking the first cell-based gene therapies for treating sickle cell disease. Growing investment and supportive government policies are also fueling market growth. In Canada, the government launched the Canadian Genomics Strategy, committing $175.1 million over seven years starting in 2024–25. This initiative aims to enhance Canada’s capacity to turn genomics research into practical applications across critical sectors. Additionally, advanced R&D facilities and a rising number of clinical trials further drive market expansion.
CRISPR growth outside north america
The Asia-Pacific region has the most incredible opportunity to experience a boost in the CRISPR technology market outside of North America. This expansion is driven by a rising prevalence of chronic diseases and a growing elderly population. In China alone, an estimated 10 million people are affected by rare diseases, highlighting the increasing demand for CRISPR-based solutions. Supportive government initiatives and growing investments are further fueling market growth. Both China and India are actively promoting genomics research and the development of cell and gene therapies. Notably, the Indian government launched the National Genome Editing & Training Center to address regional needs and support adopting various genome editing technologies, including CRISPR-Cas. Additionally, the rapid expansion of the biotechnology sector and a surge in startups are accelerating the region’s market momentum.
Leave a comment