In late 2022, Roche ended development plans for Gantenerumab, only weeks after the monoclonal antibody was unsuccessful in two Phase 3 studies. Gantenerumab was a human monoclonal anti-amyloid-beta (Aβ) antibody that lacked Roche’s Brainshullte technology. Designed to bind aggregated beta-amyloid, Gantenerumab promotes the clearance of amyloid plaques from the brain—structures believed to be central to the pathogenesis of progressive dementia. Roche announced in November 2022 that its twin studies, Graduate 1 and 2, failed to meet their primary endpoint of demonstrating that Gantenerumab could preserve cognitive and functional abilities—such as memory, problem-solving, orientation, and self-care—in patients in the early stages of Alzheimer’s disease. Roche conducted two parallel, identically designed clinical trials with approximately 1,000 participants each, who underwent physician evaluations over a period of more than two years. Participants were randomized to receive either Gantenerumab or a placebo. According to the company, the treatment resulted in a relative reduction in clinical decline of 8% in Graduate 1 and 6% in Graduate 2 compared to placebo. However, these outcomes did not reach statistical significance. Unfortunately, the quest to develop a potent drug for Alzheimer’s Disease that can not only tackle the symptoms but reverse the disease is a graveyard full of failed therapies that had countless hours of labor and enormous monetary investment behind them.
On the heels of gantenerumab failure, Roche had another card up its sleeve. That card is named Trontinemab. CEO of Roche Teresa Graham during a fourth-quarter earnings call in 2024 described the early results of trontinemab as “[trontinemab] just drops plaque like a stone”. Graham continued:
“We do know now that the removal of plaque does correlate with increased clinical benefit for these patients and so we truly believe that there is a very unique opportunity for trontinemab”
The challenge
There are several therapeutic antibodies currently in clinical development that act through mechanisms that include targeted binding to amyloid-beta (Aβ), recruitment of microglia via interactions between the antibody’s wild-type Fc domain and Fc gamma receptors, and Fc-mediated clearance of antibody–Aβ complexes. One of the most significant hurdles that has stymied all companies developing therapeutic antibodies for AD is delivering access to the central nervous system for the therapies. The blood-brain barrier, or BBB, restricts access. The BBB is a specialized characteristic of brain endothelial cells, functioning in concert with pericytes, astrocytes, and neuronal processes, and is likely a contributing factor to the limited clinical efficacy observed with current therapeutic approaches. Ensuring sufficient CNS exposure remains one of the most significant challenges in the development of effective, antibody-based disease-modifying therapies for Alzheimer’s disease and other central nervous system disorders.
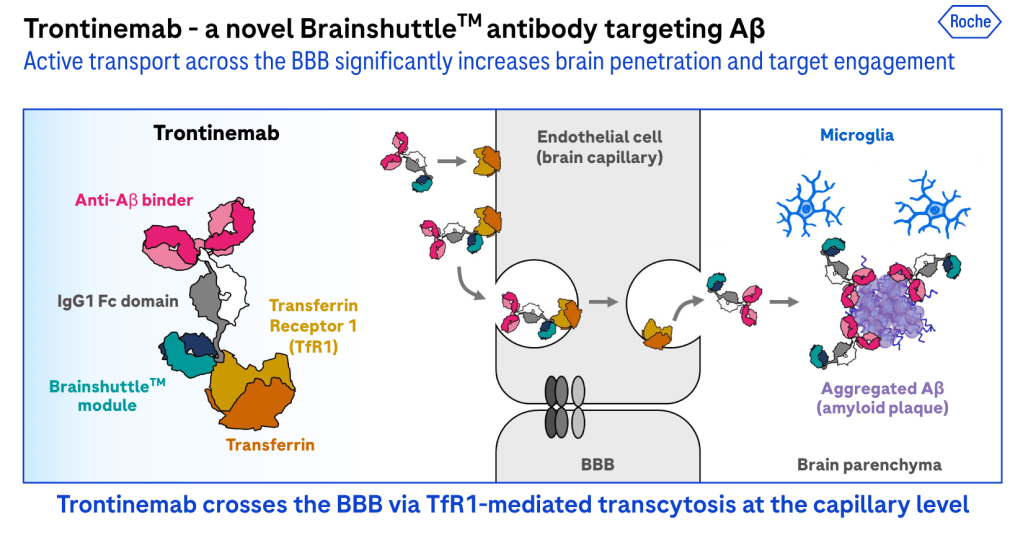
trontinemab’s moa
Researchers have investigated antibodies that bind to BBB-expressed transmembrane proteins as a means to facilitate the transport of therapeutics into the central nervous system (CNS). One of the most well-researched ones is called the transferrin receptor one or (TfR1). Using TfR1-based technology, Roche developed trontinemab – which combines gantenerumab’s complementary-determining regions (CDRs) with a novel TfR1-binding shuttle module reactive in both humans and cynomolgus monkeys. Just like gantenerumab, trontinemab binds strongly to aggregated Aβ and is thought to facilitate its removal via microglial phagocytosis. Through the Brainshuttle platform, the company aims to increase antibody concentrations in the brain while minimizing the risk of amyloid-related imaging abnormalities (ARIA).
clinical data & future directions
Preliminary results from the Phase Ib/IIa Brainshuttle AD trial, presented Thursday at the AD/PD conference, showed that trontinemab reduced amyloid plaque levels below the positivity threshold in most patients receiving the higher dose. The study enrolled 114 amyloid-positive individuals with either mild cognitive impairment or prodromal Alzheimer’s disease, who were administered trontinemab at either 1.8 mg/kg or 3.6 mg/kg every four weeks. After 28 weeks of treatment, 81% of the 26 participants in the high-dose group had their amyloid levels fall below the 24-centiloid (CL) threshold. In addition, patients exhibited ‘early and significant’ reductions in several Alzheimer’s-related biomarkers in both cerebrospinal fluid and plasma, including total tau, phosphorylated tau (pTau)181, pTau217, and neurogranin.
Along with this promising data, another key distinguishing factor that Roche showcased, setting them apart from their competitors, is the ARIA rate obtained with trontinemab. According to Roche, ARIA-E occurred in under 5% of patients (3/114), and all instances were considered radiographically mild. Their competitors posted clinical study results showing that ARIA-E occurred in 13% of patients (113/898) receiving Leqembi (lecanemab) from Biogen and Eisai and in 24% of patients (201/853) treated with Eli Lilly’s Kisunla (donanemab-azbt). This encouraging data has prompted Roche to announce the commencement of the Phase III trial for trontinemab, which is scheduled to begin later this year.
Leave a comment