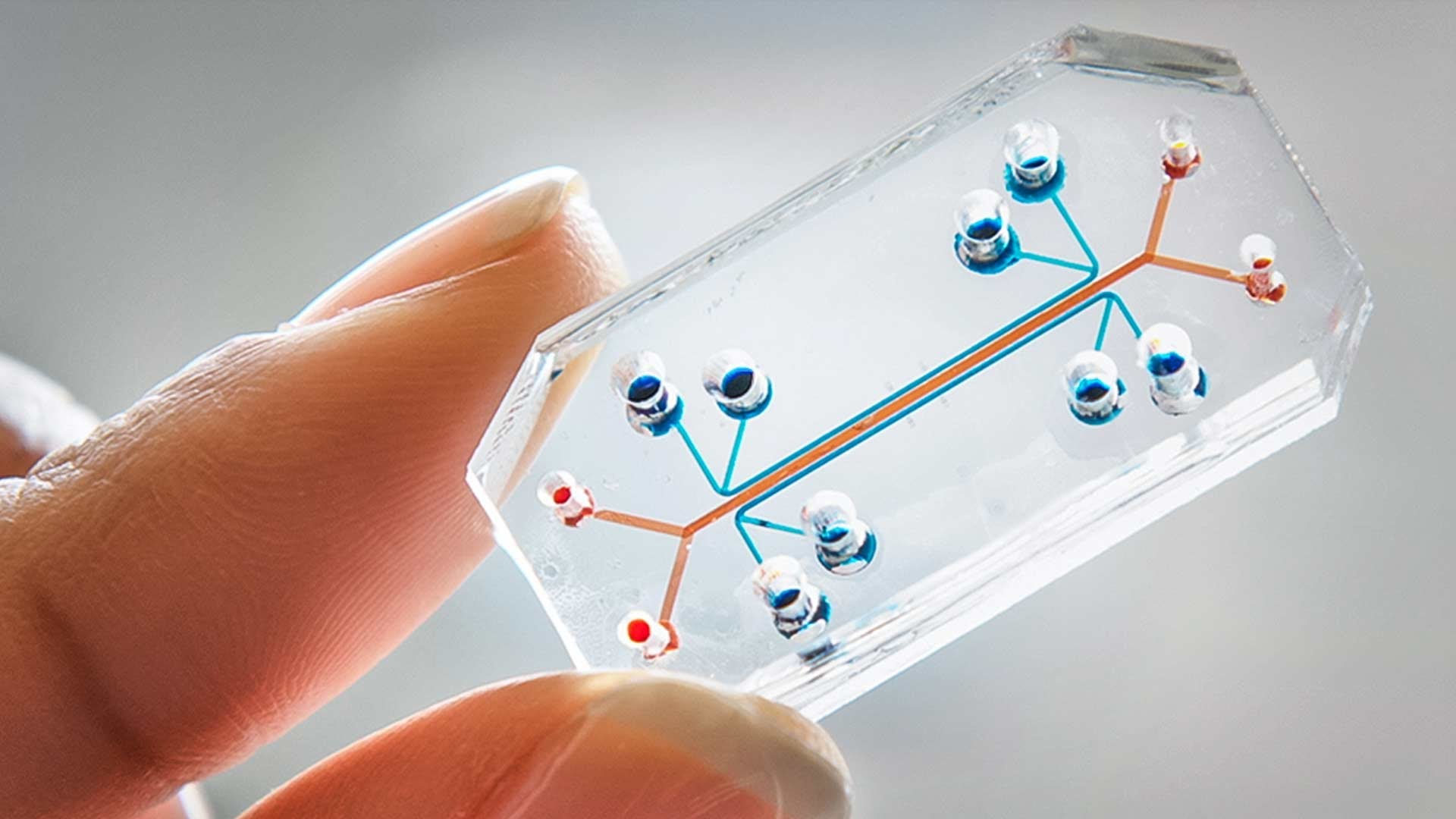
the issue
A significant portion of research in R&D for pharmaceutical and biotechnology companies focuses on understanding the disease pathology, its instigators, and the downstream effects of the disease in the human body in order to identify where a viable drug can be targeted for a cure. When examining neurodegenerative diseases, it becomes increasingly complex to accurately model disease progression in the brain in an in vitro setting. In vitro experiments are used extensively to study disease pathology and cellular functions. Animal models are then later used to validate further or explore the disease pathology and the impact of the experimental drug candidates. In the case of neurodegenerative diseases, a significant portion of the time, the data from animal models don’t align well or at all with the in vitro data generated. Not to mention animal models can also hide the potential toxicity an experimental drug can cause in humans. This mismatch in biology results in a significant loss of time, money, and human energy.
To address this gap, organ-on-chip technology is now emerging in the market. One of the most prominent players is Emulate, which in 2010 published an article in Science on the development of its lung-on-chip. Emulate was officially launched in 2014 and began advancing the development and commercialization of its Organ-Chip technology, making these cutting-edge research tools more accessible to the market. Organ-Chips are now used in over 150 laboratories worldwide, including 17 of the top 25 global biopharma companies. The FDA has utilized the technology to evaluate the safety of COVID-19 treatments and vaccines, and it has even been deployed in space research. Through strategic collaborations with companies like AstraZeneca and Johnson & Johnson, Organ-Chips are helping uncover species-specific toxicities and improve the prediction of human drug responses. Since its inception, Emulate has secured over $200 million in funding, including an $82 million Series E round led by Northpond Ventures. In recognition of its impact, Fast Company named Emulate one of the 10 Most Innovative Biotech Companies of 2022.
current research
A recent paper published in Cell Stem Cell Journal titled “An organ-chip model of sporadic ALS using iPSC-derived spinal cord motor neurons and an integrated blood-brain-like barrier” researchers from Cedars-Sinai utilized the chip technology from Emulate to essentially create “ALS on a chip”. Emulate’s chip have the capacity to be modular can be modified to create an environment to mimic various disease conditions or human organs.
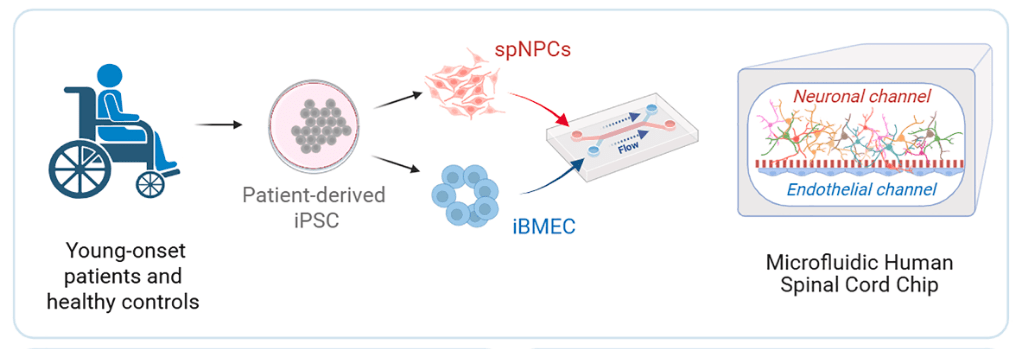
The researchers established two chip-based models, demonstrating that iPSC-derived neuronal cultures enhanced the barrier integrity and plasticity of induced brain microvascular endothelial cells (iBMECs). In contrast, the iBMECs promoted the maturation of spinal motor neurons (MNs) in turn. They hypothesized that this advanced co-culture system could more faithfully replicate MN function and reveal stronger disease phenotypes in sporadic ALS (sALS) patients.
To explore this, they developed a spinal cord motor neuron organ-chip (SC-chip). They used a combination of bulk transcriptomics, proteomics, and single-nucleus RNA sequencing (snRNA-seq) to characterize the maturation and functional features of motor neurons derived from ALS patients. The SC-chip, which incorporates continuous media flow, supports enhanced MN maturation and reveals changes in neurofilaments as well as early molecular disruptions in glutamate signaling in ALS MNs. This platform has the potential to advance our understanding of ALS pathology but also incorporates a functional, BBB-like system, offering the possibility to evaluate drug permeability and therapeutic effects in a physiologically relevant environment.
One of they key takeaways the researchers were able to confirm which has been proposed for ALS disease progression is increased glutamate signaling between neurons. This is usually altered in the ALS motor neurons. Clive N. Svendsen one of the lead researchers had this to say.
“Excessive release of glutamate has long been considered a possible cause of ALS, and one of the few drugs approved to treat the disease targets this neurotransmitter…over many years it is possible that this increased glutamate signaling may be part of why motor neurons die in ALS.”
Svendsen went on to describe the potential of the chips to further explore this one piece of the puzzle to ALS progression in patients.
“If we can show that glutamate signaling eventually makes the ALS motor neurons sick, for instance, we can apply drugs to the blood vessel side of the chip to mimic a clinical trial. Those experiments are underway.”
This research showcased the organ-on-chip and iPSC technologies, modeling an in vivo environment of young-onset sporadic ALS (sALS) that included dynamic perfusion across brain-like tissue and a functional blood-brain barrier (BBB)-like component. Researchers were able to achieve motor neuron (MN) maturation, maintain single-cell transcriptomic consistency, and identify distinct sALS molecular signatures—particularly those involving synaptic plasticity and glutamate signaling. Given that most ALS cases are sporadic and of unclear origin, this human-derived model provides a highly relevant platform for investigating disease mechanisms and advancing therapeutic development.
the past & the future
Some of the most complex in vitro experiments surrounding complex diseases involve working extensively with iPSC lines that can be utilized to grow various cells marked to play a role in the disease. For ALS, there are ALS iPSC lines, such as the ones created by Answer ALS, which stem from over 450 ALS patients. Issues arise when these cells are differentiated, don’t showcase disease signatures, and lack robust molecular phenotypes compared to controls. Organoids that mimic the structure and function of human organs are derived from stem cells. They are also commonly utilized to gain more granularity in-vitro but also come along with their limitations. Organoids are not vascularized, meaning they cannot mimic the flow of nutrients and fluids. They also often have necrotic cores, which becomes a challenge in drug development when we look to glean meaningful data from them.
The future

Players such as Emulate, who are working to commercialize organ-on-chip technology (OoCs), will become crucial to research and development for the biotech, pharmaceutical industry, and academic researchers. We recently published an article describing the FDA’s decision to move towards NAMs (New Approach Methodologies), which are emerging technologies that can replace animal studies. The organ-on-chip or disease-on-chip technology falls under the NAMs designation and holds vast potential for accelerating drug research while reducing R&D costs.
OoCs utility can be realized when combined with induced pluripotent stem cells (iPSCs) derived from individual patients, to support personalized disease modeling and tailored drug testing. This is especially valuable in rare diseases, cancer, and neurodegenerative conditions like ALS, where individual genetic differences significantly impact treatment response. OoC models can also predict drug efficacy and toxicity more accurately than traditional animal models or 2D cultures, potentially reducing reliance on animal testing. The research described above demonstrates how organ-on-chip technology is transitioning from proof-of-concept to mainstream adoption, poised to become a cornerstone of next-generation biomedical research, precision medicine, and regulatory science. As the field matures, organ-on-chip technology will not only enhance our understanding of human physiology and disease but also reshape how we develop and evaluate new therapies.
Leave a comment