AbbVie, the pharmaceutical giant, announced on June 30th that it will be acquiring Capstan Therapeutics for $2.1 billion. Capstan is a cell therapy startup that spun out of the University of Pennsylvania. The now San Diego-based Capstan utilizes messenger RNA (mRNA) to reprogram immune cells to target cells that drive disease. This mRNA is delivered using lipid nanoparticles. Unlike viral vectors—commonly used in genetic therapies but often limited to single-use due to the body’s immune response—lipid nanoparticles do not trigger such immunity, enabling repeat dosing. This ability to reduce is especially crucial for applying cell therapy in immunology, where treating chronic conditions often requires ongoing intervention. Their proprietary CellSeeker™ tLNP platform features innovative lipid nanoparticles (LNPs) linked to recombinant protein binders, such as monoclonal antibodies. These tLNPs are engineered to deliver payloads, such as mRNA or gene-editing tools, directly to specific cell types in vivo for targeted reprogramming.
“Among the diverse set of presentations showcasing Capstan’s non-viral CellSeeker™ platform, we are particularly encouraged by new preclinical data in support of our in vivo anti-CD19 CAR-T program, which demonstrate that a compact two-dose cycle was sufficient to induce rapid and deep B cell depletion in blood and tissues of non-human primates,…
These preclinical data highlight the potency of B cell depletion achievable with a transient in vivo CAR mRNA approach, without the need for lymphodepletion, and set the stage for clinical evaluation of CPTX2309.”
- Adrian Bot, M.D., Ph.D., Chief Scientific Officer and Executive Vice President of R&D at Capstan
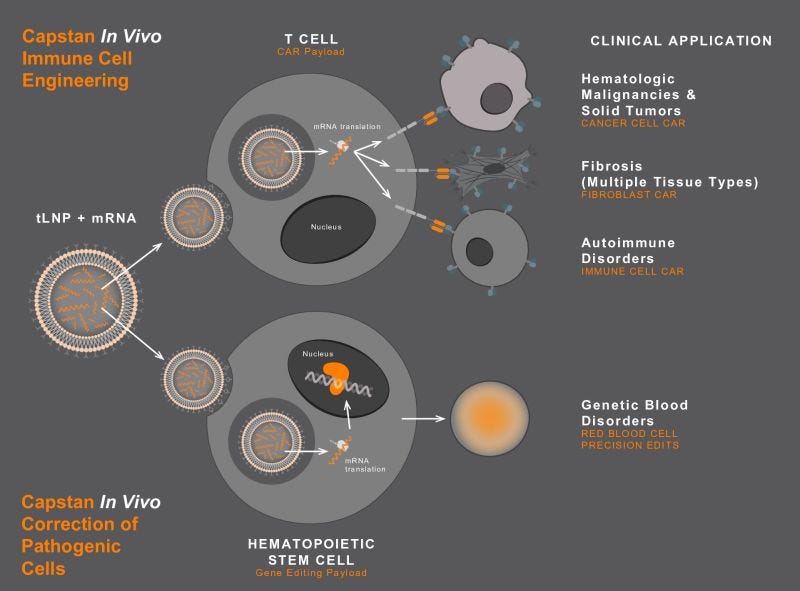
Schematic showcasing how capstan’s cellseeker technology works
what abbvie seeks to gain
There are over 100 known autoimmune diseases, such as type 1 diabetes, rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease. Capstan’s CellSeeker technology enables it to be modular, allowing for development to target a variety of autoimmune diseases. In the US alone, 4.6% of the population, or around 15 million people, suffer from some autoimmune disease, and roughly 10% of the global population suffers from the disease. This presents an excellent opportunity for Abbvie to service this patient population.
Most critically, in this acquisition, along with the CellSeeker technology, AbbVie will acquire CPTX2309, a potential first-in-class in vivo tLNP anti-CD19 CAR-T therapy currently in Phase 1 development for the treatment of B-cell-driven autoimmune diseases. B cells play a key role in the development of autoimmune diseases. CD19, a receptor found on the surface of B cells, is a clinically validated target for B cell depletion through ex vivo CAR-T cell therapy. CPTX2309, developed using Capstan’s proprietary platform with hepatic de-targeting, delivers an mRNA payload encoding an anti-CD19 CAR directly to CD8+ cytotoxic T cells. This reprogramming occurs in vivo—eliminating the need for lymphodepletion or complex ex vivo manufacturing. The modified CD8+ T cells transiently express the CD19 CAR, enabling them to target and deplete B cells in both peripheral circulation and tissues. By eliminating auto-reactive, antibody-producing memory B cells and allowing for the re-population of naïve B cells, this approach aims to reset the immune system, potentially halting disease progression and achieving clinical remission.
Abbvie holds strengths in immunology and inflammation but is seeking to expand its modality in this disease sector. Notably, Abbvie’s acquisition of Capstan comes a few days after the FDA lifted the Risk Evaluation and Mitigation Strategies (REMS) requirements for all approved autologous chimeric antigen receptor (CAR-T) cell therapies. This decision follows the removal of access restrictions, as the FDA no longer deems them necessary for this treatment modality. REMS is a safety program mandated by the FDA for medications with significant safety concerns, designed to help ensure that their benefits outweigh the risks. It restricts access to approved therapies by requiring administration only at certified hospitals and clinics equipped to provide the treatment and manage potential side effects, such as with access to anti-inflammatory medications. This is being viewed as a significant step forward in the FDA’s efforts to remove some red tape for the development of CAR-T therapies, as noted by industry leaders and Wall Street analysts.
“We view the removal of the REMS program for CAR-T cell therapies as a positive development for the space, as it supports the notion that the FDA is working to minimise red tape and increase access to potentially curative treatments for patients.”
- William Blair analysts
Abbvie’s newly acquired asset is being described as “high-risk, high-reward,” reinforcing the company’s strong market position. The pharma giant has already navigated the loss of exclusivity for its blockbuster drug Humira (adalimumab) by successfully launching Skyrizi (risankizumab) and Rinvoq (upadacitinib).
Leave a comment