
Dispatch Bio officially launched today, July 23rd, 2025, founded in 2022 with the ambitious goal of creating a universal cancer treatment. Their two-pronged approach was developed by four leading scientists — CAR-T trailblazer Carl June, M.D.; Chris Garcia, Ph.D.; Andy Minn, M.D., Ph.D.; and Kole Roybal, Ph.D. — came together to tackle some of the field’s toughest challenges, a collaboration that ultimately led to the founding of Dispatch. The biotech emerged from a partnership between the nonprofit Parker Institute for Cancer Immunotherapy (PICI) and the collective research of its four scientific co-founders, who devised a dual-pronged therapeutic strategy. Dispatch has raised a total of $216 million since its founding in 2022, fueling the advancement of its novel cancer immunotherapy platform. The Series A round was co-led by founding investors Arch Venture Partners and the Parker Institute for Cancer Immunotherapy (PICI). It attracted strategic participation from industry leaders including Bristol Myers Squibb, the University of Pennsylvania, Stanford University, and Alexandria Venture Investments.
“It’s a single product with two components,…The first component is a viral vector…It’s like spray painting cancer cells so they’re now different from the healthy cells”
- Dispatch CEO Sabah Oney, Ph.D.
At the heart of Dispatch’s approach is a synthetic protein known as the “flare” antigen, which the therapy is designed to express on all cancer cells within a patient’s body. This engineered marker enables immunotherapies to target and destroy any cell displaying the protein precisely—regardless of the cancer type or location.
flare mechanism overview
step – 1
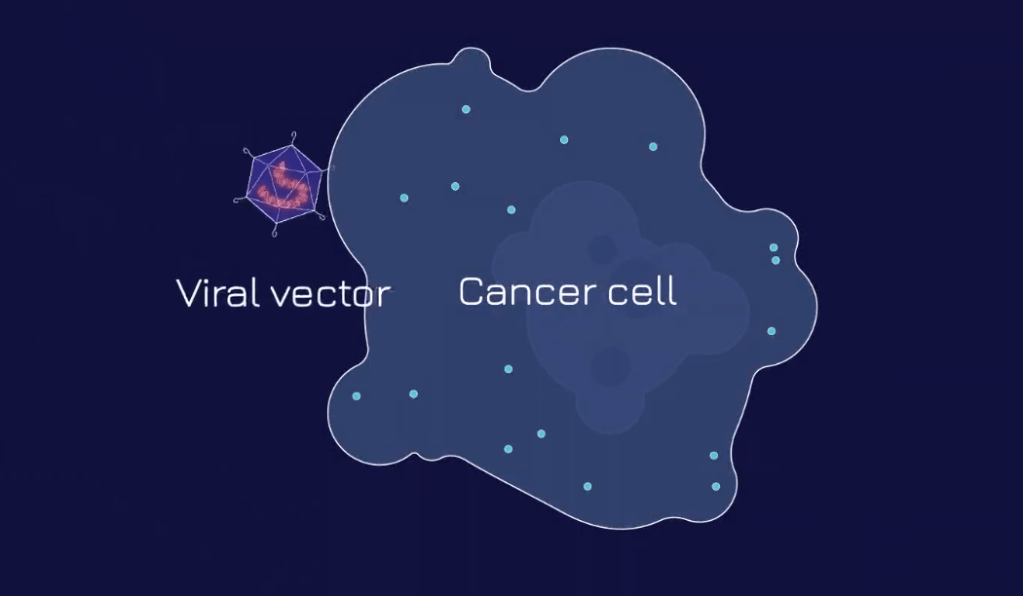
A viral vector, enclosed within the FLARE package, is introduced. The viral vector is equipped to target epithelial-derived tumors. As it approaches the target cell, the viral vector enters the cell and releases the payload. This sets up the FLARE signal on the cell surface of the cancer cell.

step – 2
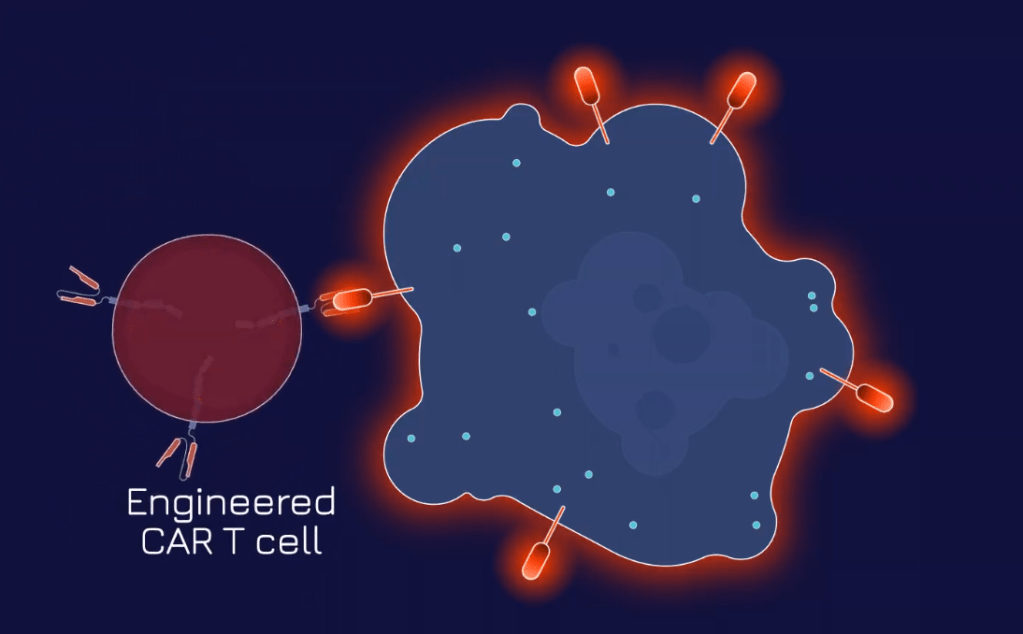
Next, Dispatch’s engineered CAR T cell is equipped to identify the FLARE antigen on tumor cells and lock in on them
STEP – 3
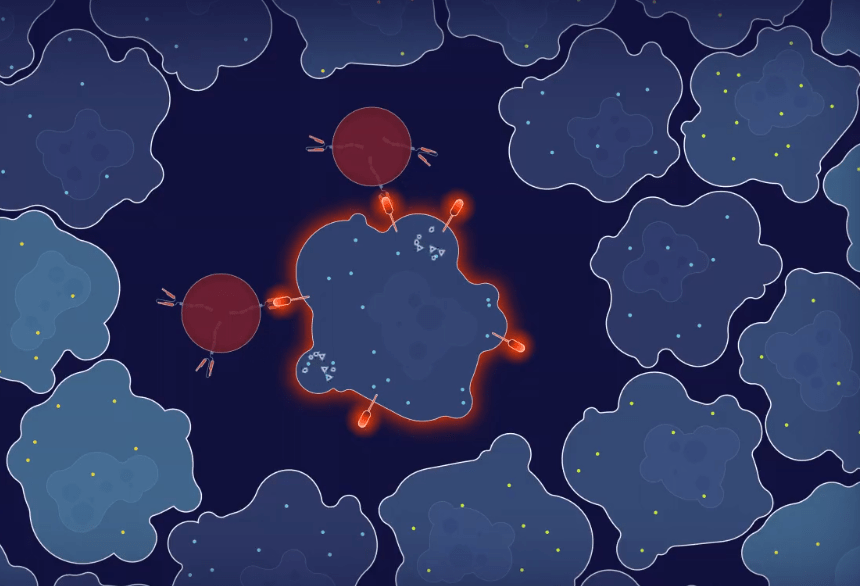
Once the CAR T cell docks on the cancer cell with the FLARE antigen, it releases the crucial proteins used by cytotoxic lymphocytes to kill the cancer cell.
STEP – 4
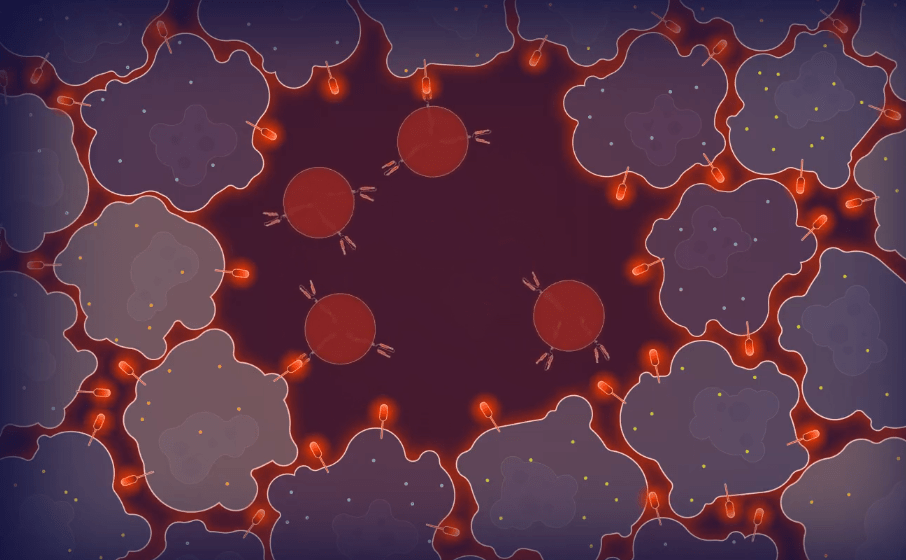
Finally, once the tumor cell is destroyed, it releases thousands of viral particles carrying FLARE into the surrounding tumor micro-environment. This results in the other tumor cells being tagged by the FLARE antigen. This allows a more efficient clearance of tumor cells across multiple sites.
What’s next for dispatch
The 60-person team at Dispatch is advancing toward IND-enabling completion for its lead candidate, positioning the company for clinical entry in the following year. Simultaneously, it is establishing a scalable manufacturing footprint through a strategic partnership with an undisclosed industry collaborator, signaling long-term commitment to end-to-end development. According to CEO Oney, Dispatch will test the technology in a homogeneous patient population to prove its effectiveness, targeting patients who are in dire need.
“The potential of this platform is really broad: It’s all epithelial cancers…But initially, we’re starting where the unmet medical need is highest. So, with this indication that we’re going to start, there’s really nothing after the first line [of treatment].”
- Dispatch CEO Sabah Oney, Ph.D.
Part of the reason Dispatch utilizes CAR T technology is that a regulatory roadmap has already been established with the FDA, making it easier for the young company to navigate clinical trials. As of mid-2025, the FDA has approved several CAR T-cell therapies, primarily targeting blood cancers, including B-cell lymphomas, acute lymphoblastic leukemia (ALL), and multiple myeloma. There are currently no approved CAR T therapies for treating solid tumors. There are checkpoint inhibitors that can activate the immune system to fight cancer, but not everyone responds to these treatments.
While CAR-T therapies have delivered strong results in hematologic malignancies, their impact in solid tumors remains limited. Solid tumors often deploy an immune-suppressive micro-environment that depletes CAR-T cell efficacy and shields cancer from immune attack. Additionally, antigen heterogeneity within these tumors poses a significant targeting challenge for single-antigen therapies. Overcoming these barriers is a key focus for next-generation cell therapy platforms. This is where Dispatch Bio’s FLARE technology, combined with CAR T cells, hopes to deliver much-needed breakthroughs – attacking cancer in a more universal capacity.
Dispatch Bio has demonstrated strong funding momentum, closing its Series A in two tranches—most recently securing $100 million last month to accelerate development. CEO Sabah Oney, a venture partner at Arch Venture Partners with leadership experience at Alector and Ariosa Diagnostics, helms the company. Its board is chaired by Jeff Marrazzo, the founding CEO of gene therapy leader Spark Therapeutics, underscoring seasoned leadership and strategic vision
Leave a comment