
Gilgamesh Pharmaceuticals has sold its lead investigational candidate, which is currently in clinical development for treating patients who are suffering from a moderate-to-severe major depressive disorder (MDD). Psychedelic medicines have recently gained attention as potential new treatments for mental health conditions like major depressive disorder, thanks to their ability to deliver fast and long-lasting relief. However, current options often come with very long and intense psychedelic experiences, which can make treatment challenging.
Bretisilocin Gilgamesh’s new type of psychedelic medicine (Clinical ID: GM-2505) is designed to overcome this hurdle. It provides a shorter, more manageable experience while still offering lasting antidepressant benefits—Bretisilocin, a novel next-generation compound that acts as both a 5-HT2A receptor agonist and 5-HT releaser. 5-HT2A receptor agonists are a type of medication that activates the 5-HT2A receptor, one of the brain receptors for serotonin (5-HT). This is the chemical that helps regulate mood, perception, and cognition. Many psychedelic substances (like psilocybin, LSD, and DMT) work mainly by activating the 5-HT2A receptor, which is why they can cause changes in mood, thinking, and sensory perception. In mental health, 5-HT2A receptor agonists are being studied as potential antidepressants because they can induce rapid and sometimes long-lasting improvements in mood — very different from traditional antidepressants, which often take weeks to work.
CLINICAL DATA OVERVIEW
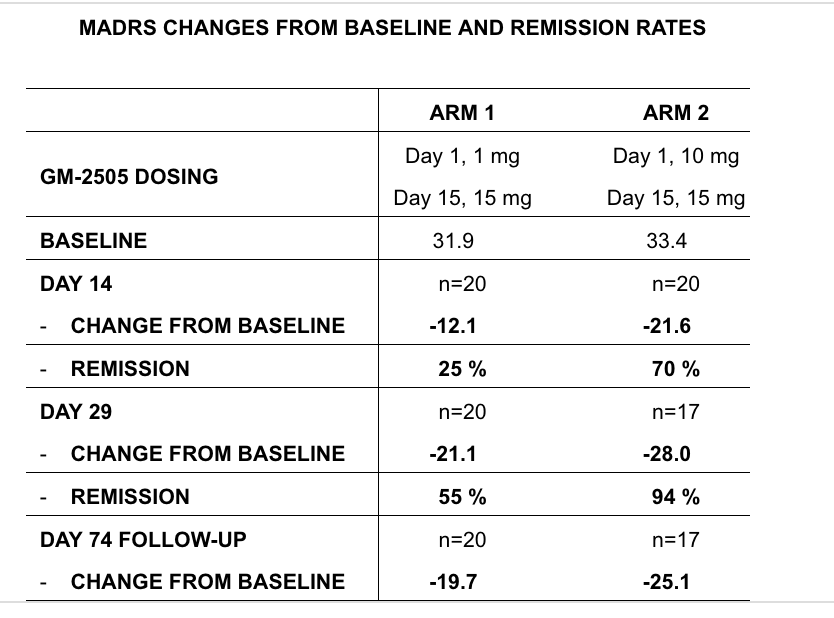
Topline results from a Phase 2a study of bretisilocin in major depressive disorder (MDD) demonstrate a clinically meaningful and statistically significant reduction in depressive symptoms versus a low-dose active comparator. At Day 14, a single 10 mg dose of bretisilocin reduced Montgomery-Åsberg Depression Rating Scale (MADRS) total score by –21.6 points from baseline, compared with –12.1 points for the 1 mg comparator (p = 0.003). Bretisilocin was generally well tolerated, with no serious adverse events reported.
“The field of psychiatry represents one of the most challenging areas in medicine, with a significant need for innovative solutions,…This acquisition underscores our commitment to broadening and enhancing psychiatric care by investing in novel treatment approaches with the potential to reach patients for whom other treatments have been ineffective. We look forward to advancing bretisilocin to late-stage clinical development.”
- Roopal Thakkar, M.D., executive vice president, research and development and chief scientific officer, AbbVie.
Terms of Agreement between AbbVie & Gilgamesh
Under the agreement, AbbVie will acquire Gilgamesh’s bretisilocin program for up to $1.2 billion, covering an upfront payment and development milestones. As part of the transaction, Gilgamesh will spin off a new entity, Gilgamesh Pharma Inc., to retain its employees and other programs, including the oral NMDA receptor antagonist blixeprodil (GM-1020), a cardio-safe ibogaine analog, the M1/M4 agonist program, and its existing collaboration with AbbVie. The transaction is subject to customary closing conditions.
This deal builds on AbbVie and Gilgamesh’s 2024 collaboration and option-to-license agreement for the development of next-generation therapies for psychiatric disorders. The existing option-to-license will remain in effect and will transfer to Gilgamesh Pharma Inc. following the spin-out. Under the agreement, AbbVie and Gilgamesh will collaborate to research and develop a portfolio of next-generation therapeutics for psychiatric disorders. If AbbVie exercises its option, it will assume responsibility for development and commercialization. Gilgamesh will receive a $65 million upfront payment. It may earn up to $1.95 billion in total option fees and milestone payments, in addition to tiered royalties ranging from mid-single digits to low-double digits on net sales.

Leave a comment